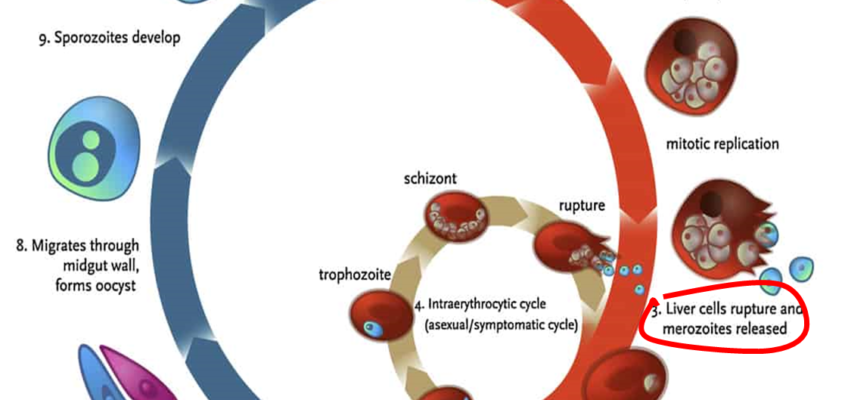
Malaria causes over 400,000 deaths annually and impacts the lives of over two billion people worldwide damaging family livelihoods and national economies (World Health Organization, 2020). There were 229 million new cases in 2019 (World Health Organization, 2020). Major efforts in recent years have resulted in Malaria elimination in several countries. However, drug resistance is a major threat to elimination, and there are signs of malaria parasite resistance to the Artemisin-based combination therapies (ACT), currently utilized in Africa (Rosental, 2021; Tse, Korsik, & Todd, 2019). The drug ACT is the first-line treatment for all forms of malaria in a majority of countries endemic for malaria. Of particular concern is that failure of ACT will result in untreatable falciparum malaria (the most lethal form of malaria).
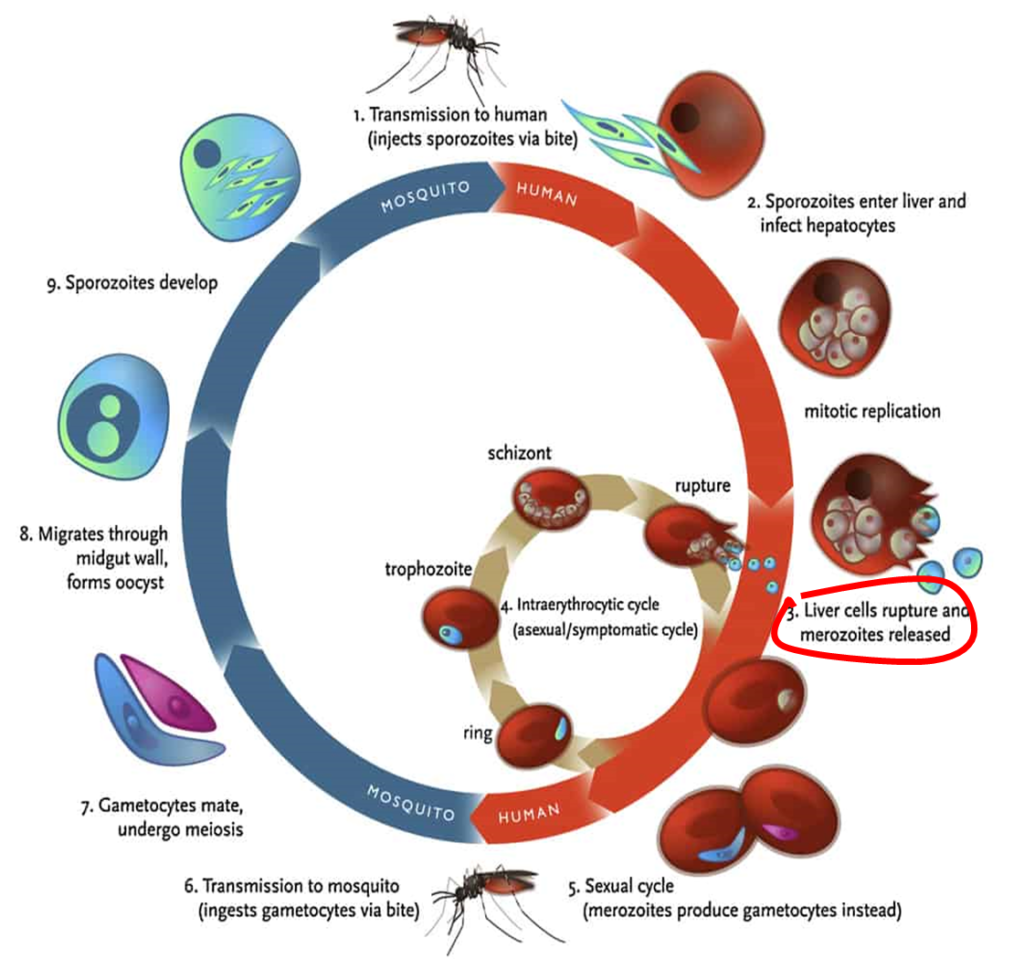
In 2020, a phase two research trial in Vietnam revealed that Imatinib, a repurposed anti-cancer drug utilized in combination with ACT, is 100% effective in treating malaria (Chien et al., 2021). Imatinib works by targetting a red blood cell enzyme, therefore, blocking the emergence of the Merozoite form (see Diagram 1, number 3 shows Merozoites released from the liver cells) of the Malaria parasite into the blood stream, leaving it to disintegrate without mutating (Kesely, Pantaleo, Turrini, Olupot-Olupot, & Low, 2016).
100% of the Malaria parasites were cleared within three days meaning that there was no drug resistance, and much less transmission (Chien et al., 2021).
Chien et al (2021) found that patients who received Imatinib did not experience extra toxicity, recovered much faster from Malaria symptoms and that all Malaria parasites were cleared within three days, meaning that there was no drug resistance and much less transmission. The drug is also cheap to produce.
Limitations of the research are that only adult males could take part (NIH US National Library of Medicine, 2021). Pregnant women and children are the populations most at risk from Malaria. However, it would have been unethical to have involved women of reproductive age because Imatinib is known to cause foetal abnormalities, preterm and still births in laboratory animals (Drugs.com, 2021; MEDSCAPE, 2014). Nonetheless, Imatinib shows promise as a component of Malaria treatment as the patients involved experienced few side effects and good parasite clearance. Phase three research is to be carried out in Vietnam in the near future where multidrug resistance, including ACT, is the biggest threat to malaria control and elimination, and to other countries in the Greater Mekong Subregion.
References
Chien, H. D., Pantaleo, A., Kesely, K. R., Noomuna, P., Putt, K. S., ., Tuan, T. A., . . . Turrini, F. M. (2021). Imatinib augments standard malaria combination therapy without added toxicity. Journal of Experimental Medicine, 218(10). doi:10.1084/jem.20210724
Drugs.com. (2021, August 31). Amatinib. Retrieved from https://www.drugs.com/monograph/imatinib.html
Kesely, K. R., Pantaleo, A., Turrini, F. M., Olupot-Olupot, P., & Low, P. S. (2016). Inhibition of an Erythrocyte Tyrosine Kinase with Imatinib Prevents Plasmodium falciparum Egress and Terminates Parasitemia. PloS one, 11(10), e0164895. doi:10.1371/journal.pone.0164895. (Accession No. 27768734)
MEDSCAPE. (2014). imatinib (Rx) – Gleevec. Retrieved from https://web.archive.org/web/20140103224530/http://reference.medscape.com/drug/gleevec-imatinib-342239#showall
NIH US National Library of Medicine. (2021). Effect of Imatinib on Suppression of Malaria Parasites in Patients With Uncomplicated Plasmodium Falciparum Malaria (MIM). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02614404. (NCT02614404). Retrieved September 21, 2021 https://clinicaltrials.gov/ct2/show/NCT02614404
Rosental, P. J. (2021). Editorial: Are Artemisinin-Based Combination Therapies For Malaria Beginning To Fail in Africa? American Journal of Tropical Medical Hygiene, 105(4), 857-858. doi:10.4269/ajtmh.21-0797
Tse, E. G., Korsik, M., & Todd, M. H. (2019). The past, present and future of anti-malarial medicines. Malaria Journal, 18(1), 93. doi:10.1186/s12936-019-2724-z
World Health Organization. (2020). World Malaria Report (WMR) 2020: Complete Analysis of the WHO Report: World malaria report 2020: 20 years of global progress and challenges (ISBN-978-92-4-001579-1 (electronic version)). Retrieved from https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020/