Précised from Zarocostas, and adapted by Dr Kerre Ann Willsher and Dr Jenny Kerrison
Since 2015, malaria cases and deaths have increased. Progress in malaria elimination has stagnated (Paaijmans & Lobo, 2023). With two new malaria vaccines developed, there is hope that malaria elimination regains its momentum. Gavi, the Vaccine Alliance maintains that the major barrier to the distribution of malaria vaccines in endemic countries is supply (Gavi The Vaccine Alliance, 2023; Zarocostas, 2023).
Gavi released its roadmap of requirements for efficient malaria vaccine distribution on April 25th, 2023, World Malaria Day. Gavi (2023) outlined that 40-60 million doses of malaria vaccine are required by 2026, increasing to 80-100 million doses by 2030. A programme with an inaugural investment of US$155.7 million dollars has been mapped for eligible sub-Saharan African countries.
Priorities in the Roadmap include the reduction of the price of malaria vaccines, increasing the supply, and manufacturing in Africa, all of which require different forms of capital and incentives (Botting cited in Zarocostas, 2023). The first malaria vaccine RTS.S/AS01 for Plasmodium falciparum has been piloted among children 5 to 17 months of age in Ghana, Kenya, and Malawi since 2019, The vaccine was the product of 30 years of research and development by Glaxo Smith Kline (GSK) in partnership with PATH.
In October 2021, the World Health Organization (WHO) then made its first prequalification of a malaria vaccine with a recommended schedule of four doses of RTS.S/AS01 for children in sub-Saharan Africa and other regions where Plasmodium falciparum transmission is moderate to high. The vaccine is recommended as one of several tools in a comprehensive approach to malaria control (World Health Organisation, 2023, p. 143) Prequalification started an avalanche of demand with 29 countries indicating to Gavi that they would like to introduce the vaccine (Hamel cited in Zarocostas, 2023).
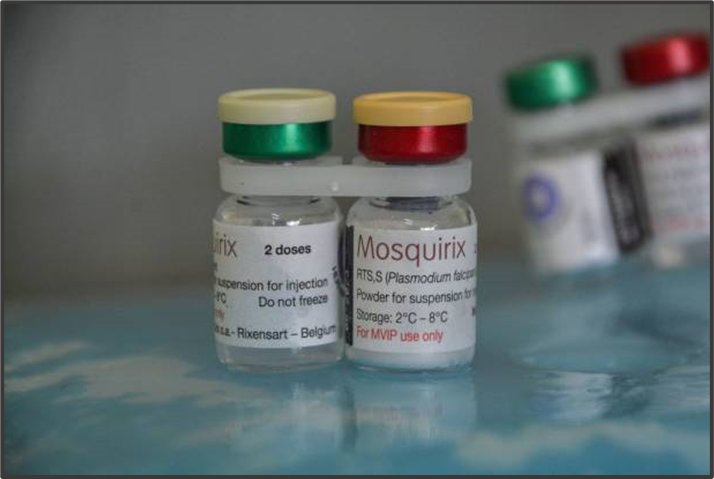
In August 2022, UNICEF obtained 18 million doses of RTS.S/AS01 with funding from Gavi over three years at 9.30 euros per dose. GSK will increase the manufacture of RTS.S/AS01 to 15 million doses per year during 2026-2028 via a partnership transfer with the Indian company Bharat Biotech. This supply appears to be a drop in the bucket and expensive, but while supplies are limited, WHO will prioritize the countries with overriding need. There is also hope that the likely prequalification by WHO of R21/Matrix-M, a second malaria vaccine will result in greatly reduced costs of malaria vaccine and much improved supplies. R21/Matrix-M will be manufactured by the Serum Institute of India which has the capacity to produce 100-200,000 doses at US$3.00 or less annually. Therefore, the Serum Institute of India will most likely dominate the market for a time as other vaccines are still in the beginning stages.
The Gavi roadmap is robust, but given the considerable demand for the malaria vaccine, “the biggest hurdle will be to have sufficient resources to execute the plan quickly” according to Botting (cited in Zarocostas, 2023). Therefore, collaboration is required at all levels. Malaria elimination requires a comprehensive approach. As highlighted by Sands, Executive Director of the Global Fund (cited in Zarocostas, 2023), healthcare systems with grassroots facilities and community health workers are essential as well to ensure that the supplies quickly get to the children in most need, often the poorest, marginalized, and hardest to reach.
Optimal combinations of malaria prevention strategies including management of mosquito breeding sites, insecticide-treated nets, and vaccines are necessary. Supply is just one part of the equation in malaria prevention.

References
Gavi The Vaccine Alliance. (2023). Malaria vaccine market shaping roadmap [White Paper]. 8. Retrieved May 18, 2023, from https://www.gavi.org/sites/default/files/white-paper/WPaper_malaria-roadmap.pdf
Paaijmans, K. P., & Lobo, N. F. (2023). Gaps in protection: the actual challenge in malaria elimination. Malaria Journal, 22(46). https://doi.org/10.1186/s12936-023-04473-x
World Health Organisation. (2023, March 13, 2023). Guidelines for Malaria The United Nations. Retrieved June 1, 2023 from https://www.who.int/publications/i/item/guidelines-for-malaria
Zarocostas, J. (2023). Gavi unveils malaria vaccine plans. The Lancet, 401(May 6, 2023), 1485. www.lancet.com

